


Discoloration caused by silver corrosion often appears amber and can accompany the fading of the image, as seen in this 35mm nitrate print. Oxidation of silver has been promoted by the decay of the nitrate base. The gelatin in this sample is also soft and sticky, partially decomposed by the oxidizing compounds and acids products of nitrate decay.

The images on this tinted 35mm nitrate newsreel have faded. Nitrate decay produced nitrogen oxides and strong acids that have attacked the image area.

Elsewhere in the same 35mm nitrate newsreel shown in Image 77, a section tinted with a different dye has faded further, demonstrating that different parts of reels often fade and discolor at different rates. Here the images have faded almost entirely.

The brown discoloration present in this strip of 35mm nitrate has been caused by the oxidation of silver in the gelatin binder.

In this example, 35mm black nitrate leader has discolored and then faded entirely in places due to silver corrosion.

Fading and discoloration in this 35mm nitrate dupe negative has been promoted by the decay of the nitrate base, which has also partially decomposed the gelatin, which is soft and sticky.
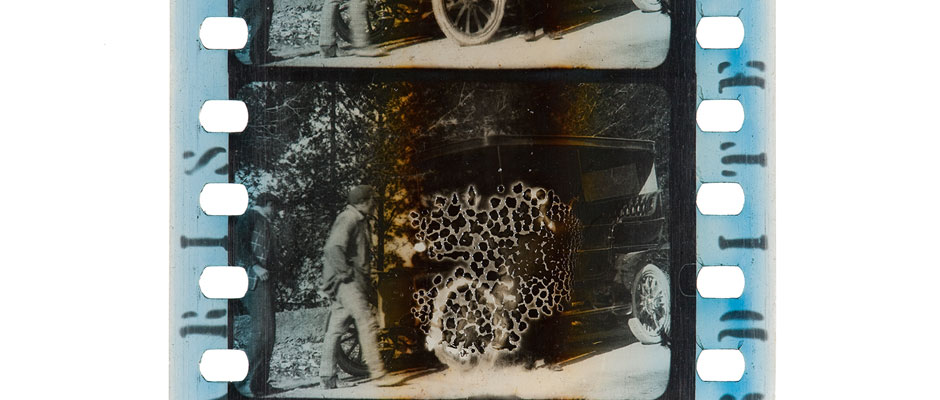
Brown discoloration in this frame of 35mm has been accelerated by nitrate base decay. It is accompanied by other signs of nitrate decay, including bubbling, partially decomposed gelatin and fading of the blue dye tint. Notice the difference in the blue dye from the edges of the film to the center of the image, where it has completely faded.
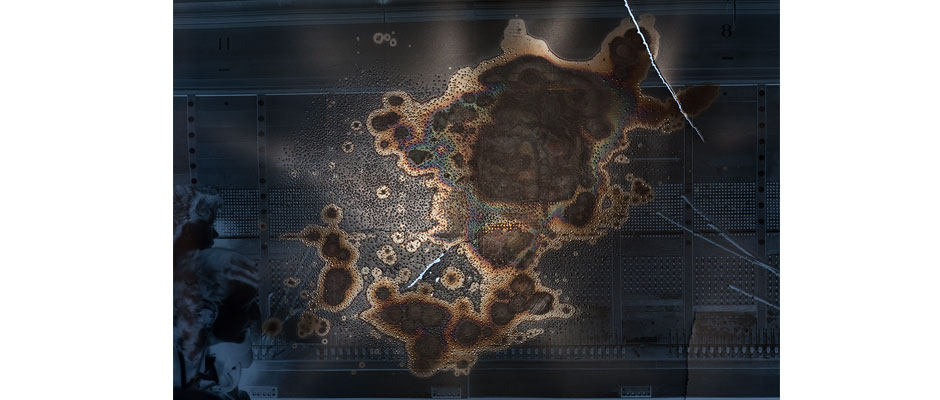
Acetate support decay, unlike nitrate support decay, does not produce oxidizing compounds. But silver images in acetate film are still subject to oxidation via moisture or pollutants in the atmosphere. In this case, an acetate negative displays silver fading and mirroring after having been stored against a decaying nitrate negative. Oxidizing compounds released by the decaying nitrate negative attacked the silver image in the acetate negative, producing levels of corrosion not normally seen in acetate film. Decaying film should always be segregated from other film materials, and decaying nitrate, if left stored with acetate, will promote acetate decay, oxidation, and dye fade in those materials.
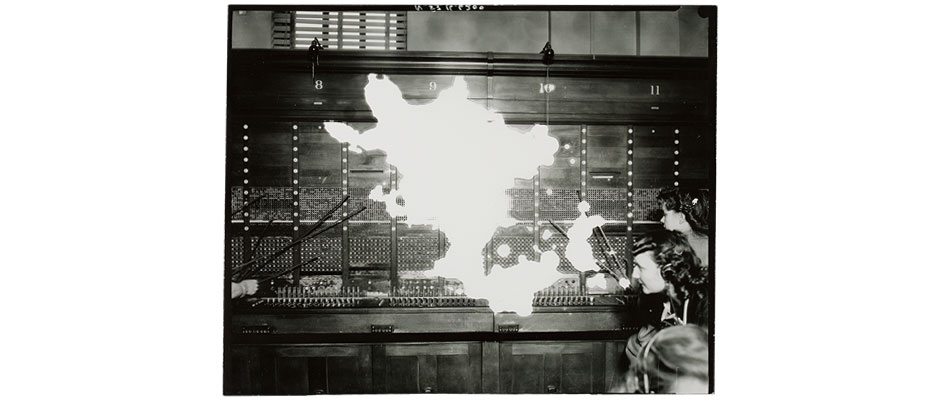
This photographic print was produced from the decayed acetate negative seen in the previous image. The oxidation in the silver image has resulted in partial loss of the image when printed.
What it is and what causes itImages on black and white film are typically formed by metallic silver in a gelatin binder. When exposed to a combination of moisture in the environment and pollutants in the air or contaminants in the film’s enclosure, this image silver will corrode.Image-forming metallic silver exists in a filamentary structure. When corrosion occurs, oxidative gases permeate the gelatin and attack small sites of silver, severing them from the filamentary structure and converting them into silver ions. The silver ions migrate through the gelatin and reduce as very small particles of colloidal silver, redepositing in new locations. Corrosion causes changes in the tone of the image, including fading, as oxidation attacks the filamentary image silver and decreases density. Corrosion also causes discoloration of the image because the reduction reaction produces colloidal silver, a small particle with a brown/red color. Discoloration of film can appear yellow, amber, or brown. Fading and discoloration are common in nitrate base film because nitrate base decay produces strong oxidizing compounds. Other effects of corrosion include silver mirroring and redox blemishes. While dramatic corrosion is most frequently seen in nitrate film, acetate and polyester base films are also susceptible to corrosion, since any gelatin emulsion containing metallic silver will corrode if moisture and pollutants are present in the environment. |
What you can doMoisture in the air is the critical factor that causes oxidation of the silver image, so prevention of corrosion requires an environment with controlled relative humidity, preferably between 20-50%. Storage of film in an environment free of contaminants and in inert enclosures is also recommended for prevention of silver oxidation. A low temperature environment is also preferable, but is not as important in the prevention of oxidation as is the control of relative humidity or reduction of possible contaminants in the film’s enclosures. |
At Risk
|