


The cyan and yellow dyes in this 35mm print have faded heavily, leaving only the magenta dye layer.

Notice the loss of detail of this faded 35mm print. Loss of detail is particularly evident in the highlights of the image. The presence of magnetic soundtracks on this print may have hastened acetate decay, which in turn accelerated dye fading. Magnetic coated films are particularly susceptible to acetate decay.
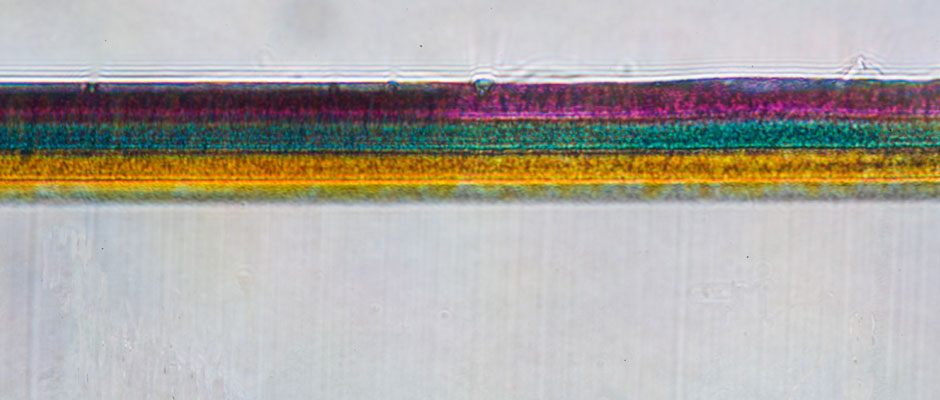
A magnified cross-section of a 35mm chromogenic color print shows the magenta, cyan and yellow dye layers. Each dye layer contains dye clouds that form the image. The dye layers will spontaneously fade in the dark at different rates, compromising color balance, contrast, and reducing detail in the image. For Eastmancolor negatives and prints made between 1950 and 1981, the cyan and yellow dyes fade more rapidly than the magenta layer.

At 50x magnification, the magenta, cyan, and yellow dye clouds in this 16mm Kodachrome chromogenic reversal film are visible.
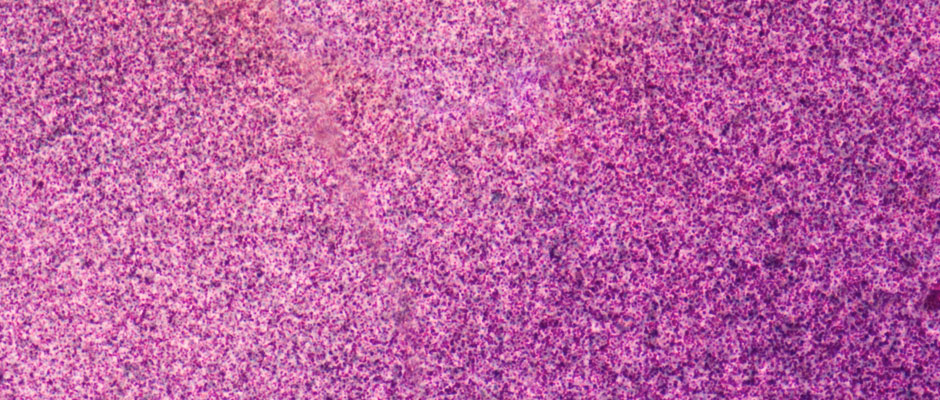
This faded chromogenic print, viewed at 50x magnification, reveals a yellow dye layer that has entirely faded. Dye fade is irreversible: when a dye fades from a film print, the image information contained by the dye is lost.

A frame of unfaded 35mm motion picture film (Right) is compared to a frame that has been subjected to accelerated aging in the laboratory, causing dye fade (Left). In this case, the magenta layer has faded.

The dye fading in the magenta layer of this chromogenic transparency may be partly caused by light fading. Although dark fading is the prime cause of color fade in chromogenic film materials, slide film which has been projected for more than a few hours can demonstrate fading caused by projection lamps. For chromogenic slide films, light fading often fades the magenta dye layer faster than the other layers. Light fading is not a concern for motion picture film because of the very short periods each frame of film is illuminated by the projector lamp.

Different film stocks and periods of manufacture have different fading characteristics. These differences are related to the varying dye couplers employed in the manufacture of chromogenic emulsions, which produce dye clouds that fade at different rates.
What it is and what causes itMany of the dyes used in color films are unstable organic compounds. Natural aging of dye compounds results in the breaking of the bonds between atoms in the dye molecules, causing dyes to fade. Dye fading reduces the overall density of the image, which results in loss of contrast. Additionally, since different dyes have different stability characteristics, color dyes fade unevenly, resulting in distracting shifts in color balance.Dyes are susceptible to both light fading and dark fading, but light is not necessary to cause dye fade in film materials. The fading that devastates chromogenic films occurs in the dark, and is accelerated by higher temperatures and, to a lesser extent, relative humidity. Both acetate and nitrate base decay may contribute to dye fade by lowering the pH of the gelatin binder, promoting dye fade in pH-sensitive dye layers. The dark stability of chromogenic films varies considerably among film emulsions and the time of the film stock’s manufacture. For instance, Kodacolor films from the period 1942- 1953 are known to have remarkably poor dye stability in the dark and exhibit intense yellow/orange staining. Kodak Ektachrome films introduced in the early 1950s are dramatically less stable than the Kodachrome films they replaced. For 35mm motion picture films, Kodak Eastmancolor negatives and prints from 1950 onwards exhibit poor dark stability, especially in comparison with their main competitor in the color feature film market, Technicolor. Technicolor films employed a proprietary dye transfer printing process with very stable dark fading characteristics. From the 1960s, however, chromogenic films predominate, and Technicolor prints became more and more scarce. From the mid 1980s onwards, chromogenic films achieved the best dark stability possible for the process, though dye stability of chromogenic films still does not match that of dye transfer processes. |
What you can doDye fading is irreversible. Once the dye images have faded, the information lost cannot be recovered. However, digital color restoration techniques may approximate the original color balance and simulate the original color information.Although acetate base decay will accelerate dye fading, all color films (nitrate, acetates, and polyesters) are vulnerable to dye fading in the dark. For polyester color films, the color dyes in the gelatin binder are the limiting factor in the longevity of the materials. Cold or frozen storage environments and moderate relative humidity will slow the progress of dye fading. The temperature at which color materials are stored is the critical factor in arresting the progress of dark fading. |
At Risk
|